i-Diagnostics Applications
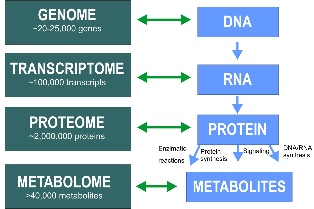
Traditional molecular diagnostics devices detect biological markers either in the genome, transcriptome, proteome, or metabolome. While DNA and RNA determine how the cells express their genes and produce proteins, proteins essentially rule the cells and tissues, producing metabolites – the products of biological processes. In the individual, the complex of these molecules provides critical information to health: it can identify the disease (diagnose) and project the dynamics of future changes (prognose). To obtain the complete picture needed for diagnosis and to make a prognosis, one needs to detect all four classes of biomarkers. This aggregate of data improves the ability to better diagnose, monitor, or predict disease, detect risk, and decide which therapies will work best for the individual patient [1, 2]. Everyone is truly unique; we respond differently to the same drugs and to the same nutrients. By analyzing the specifics of the patient and their response, molecular diagnostics offers the prospect of personalized medicine [3].
While typical methods of molecular diagnostics detect only one class of biomarkers, as mentioned above, i-Diagnostics combines detection over four classes of biomarkers: DNA, RNA, proteins, and metabolites. This unique feature makes i-Diagnostics open to the broadest range of applications. i-Diagnostics tests will be useful in a range of medical applications, including infectious disease, oncology, human leucocyte antigen typing (which investigates and predicts immune function), coagulation, and pharmacogenomics-the genetic prediction of which drugs will work best [4].
Applications for i-Diagnostics will include tests for:

- Prevention pandemics and epidemics
- Detection and diagnosing of infection diseases (COVID-19, influenza, Ebola, HIV, Zika, STDs, etc)
- Diagnosis and prognosis of cancer
- Diagnosis and prognosis of cardiovascular diseases
- Diagnosis and prognosis of Alzheimer’s, Parkinson’s and other neurological disorders
- Drug development studies
- Longevity studies and popular efforts
- Food and water safety applications
- Civil and military biodefense applications
- Forensic applications
- Environmental applications
- Agricultural analyses and studies
We believe that many routine analyses of blood, urine and other bodily fluids that currently are performed in clinical labs, will migrate to i-Diagnostics, because of the convenience and privacy of home use tests. Along with the main goal of home use, family doctors, cardiologists, dentists, first responders, pharmaceutical companies, food safety, agriculture, and environment protection specialists have expressed their interest in using i-Diagnostics for their applications. There are thousands of new applications that can be developed for the i-Diagnostics platform. It is too difficult, if not impossible, for a single company to develop all of these numerous applications. Therefore, we invite other research groups worldwide to join our team to interface their existing tests and develop new applications with the i-Diagnostics platform using the Open Innovation Business Model.
To facilitate these efforts, we are offering the uTIRF Biodetection Station and i-Diagnostics Application Development Kit (ADK). We anticipate a large demand for uTIRF-ADK, because there are tens of thousands of research groups worldwide that develop diagnostic assays, discover and validate molecular markers, and investigate the molecular mechanisms of related biological processes. These research applications will pave the way for medical use of i-Diagnostics.
In our laboratory based in North Carolina, our team is developing diagnostic and prognostic tests for prostate cancer. We have developed i-Diagnostics tests for food safety and detecting allergens in food. Our long-term collaborator Dr. Vaca from the Institute of Cellular Physiology at UNAM and his group are developing preemptive diagnostic tests for Alzheimer’s disease and hepatitis C. His colleagues from other research groups will develop tests for pancreatic and colon cancer. Dr. Dong from Washington State University is developing tests for cardiovascular diseases. The Laboratory of Molecular Virology at the Food and Drug Administration have expressed their interest in using i-Diagnostics for the rapid detection of HIV and other infectious diseases.
There are hundreds of other exciting applications that will be completely new for the healthcare area. In our laboratory, we are also working on longevity tests. These studies promise rapid progress in lengthening the average life span, and preventing metabolic disbalances that result in premature aging. From personalized precision medicine, clinical and pre-clinical tests of new drugs, and disease risks assessment to studies in food and water safety, detection of allergens, and other environmental and agricultural studies, i-Diagnostics offers the versatility for applications in all of these fields and more.
LITERATURE CITED
- Poste G (May 2001). "Molecular diagnostics: a powerful new component of the healthcare value chain". Expert Review of Molecular Diagnostics. 1 (1): 1–5. doi:10.1586/14737159.1.1.1. PMID 11901792.
- Burtis CA, Ashwood ER, Bruns DE (2012). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. Elsevier. ISBN 978-1-4557-5942-2.
- Hamburg MA, Collins FS (July 2010). "The path to personalized medicine". The New England Journal of Medicine. 363 (4): 301–4.doi:10.1056/NEJMp1006304. PMID 20551152.
- Grody WW, Nakamura RM, Strom CM, Kiechle, Frederick L. (2010). Molecular Diagnostics: Techniques and Applications for the Clinical Laboratory. Boston MA: Academic Press Inc. ISBN 978-0-12-369428-7.
Risks of Natural Pandemics/Epidemics and Biological Technologies
In 2016, the President’s Council of Advisors on Science and Technology (PCAST) issued a warning concerning the risks of naturally occurring and man-made pathogens [1]. In this letter, PCAST recommended “strengthening a national laboratory network for pathogen surveillance to spot and identify these potential risks early.” The PCAST’s letter noted: “Just as rapid advances in biotechnology have increased the risk of misuse by bad actors, they have expanded the tools available to protect the public.” Although DNA manipulation, CRISPR, synthetic biology and other biotech tools promise to cure diseases and improve many other aspects of our life, if used by bad actors, these powerful tools will impose imminent risks to the public [2-4]. PCAST was not alone in warning against a future pandemic, it was widely expected by many in the scientific community [5] [Bill Gates’ TED video].
The COVID19 pandemic has highlighted the weakness of our biodefense infrastructure. It is clear that our efforts have been more reactive than proactive. Currently, diagnostics are in high demand and unavailable to many. Today, we wait a day or two to know whether we are COVID19 positive or negative. As a result, thousands of people could be infected before the results are available. The delays and the costs of testing are increasing mortality. It is up to all of us to be prepared for the future, lest we forget or ignore the next biological threat.
After 9-11 and the anthrax letters attack, scientists warned the U.S. government about the necessity of massive coverage diagnostics and a knowledge-based biological safety infrastructure [1-5]. However, the concerns of scientists were frequently perceived as a doomsday panic.
The number of confirmed cases for the novel coronavirus disease COVID-19 officially issued by countries and widely commented on by national and international media outlets dramatically understates the true number of infections, a recent report from the University of Göttingen suggests. Dr. Christian Bommer and Professor Sebastian Vollmer from Göttingen University have used estimates of COVID-19 mortality and time until death from a recent study published in The Lancet Infectious Diseases to test the quality of official case records.
Their data shows that countries have only discovered on average about 6% of coronavirus infections and the true number of infected people worldwide may already have reached several tens of millions. Insufficient and delayed testing may explain why some European countries, such as Italy and Spain, are experiencing much higher casualty numbers (relative to reported confirmed cases) than Germany, which has detected an estimated 15.6% of infections compared to only 3.5% in Italy or 1.7% in Spain. Detection rates are even lower in the United States (1.6%) and the United Kingdom (1.2%) — two countries that have received widespread criticism from public health experts for their delayed response to the pandemic.
In sharp contrast to this, South Korea appears to have discovered almost half of all its SARS-CoV-2 infections. The authors estimate that on 31 March 2020, Germany had 460,000 infections. Based on the same method, they calculate that the United States had more than ten million, Spain more than five million, Italy around three million and the United Kingdom around two million infections. On the same day, the Johns Hopkins University reported that globally there were less than 900,000 confirmed cases, meaning that the vast majority of infections were undetected.
Sebastian Vollmer, Professor of Development Economics at the University of Göttingen, says, “These results mean that governments and policy-makers need to exercise extreme caution when interpreting case numbers for planning purposes. Such extreme differences in the amount and quality of testing carried out in different countries mean that official case records are largely uninformative and do not provide helpful information.” Christian Bommer adds: “Major improvements in the ability of countries to detect new infections and contain the virus are urgently needed.”
Is it rational to spend resources to study and mitigate the risks of human extinction from biological attacks? The risks of such a catastrophe are assumed to be low, so a skeptic might argue that mitigating such risks would be a waste of resources [8]. Diseases have been responsible for the greatest death tolls on humanity. In 1918, flu was responsible for more than 50 million deaths, while smallpox killed ~10 times that many in the 20th century. The Black Death plague was responsible for killing over 25% of the European population, while other pandemics, such as the plague of Justinian, are thought to have killed 25 million in the 6th century—constituting over 10% of the world’s population at the time. It is an open question whether a future pandemic could result in outright human extinction or the irreversible collapse of civilization.
Progress of biological technologies promises of transforming the way the world produces food and portable fuels, protects the environment, and treats disease [1]. While the progress of biotechnology is a great benefit for society, it also holds serious potential for destructive use by both states and technically-competent individuals with access to modern laboratory facilities. Since 2001, the US government has spent billions of dollars annually to protect the Nation against both intentional biological attacks and emerging infectious diseases, and much has been accomplished. But, molecular biologists, microbiologists, and virologists can look ahead and anticipate that the nature of biological threats will change substantially over the coming years – in ways both predictable and un-predictable. The US government’s past ways of thinking and organizing to meet biological threats need to change to reflect and address this rapidly-developing landscape.
LITERATURE CITED
- PCAST Letter to U.S.President (PCAST - President’s Council of Advisors on Science and Technology), “Action needed to protect against biological attack,” November 2016. https://obamawhitehouse.archives.gov/blog/2016/11/15/pcast-letterpresident-action-needed-protect-against-biological-attack.
- Turchin A, Green BP, Denkenberger D. Artificial Multipandemic as the Most Plausible and Dangerous Global Catastrophic Risk Connected with Bioweapons and Synthetic Biology. 2018. https://philpapers.org/rec/TURAMA-3
- Sotos JG, “Biotechnology and the lifetime of technical civilizations.” Cornell University Library, 2017 arXiv.org :1709.01149v1 [physics.pop-ph].
- Cooper J, “Bioterrorism and the Fermi Paradox,” International Journal of Astrobiology, vol. 12, no. 2, pp. 144–148, 2013.
- Bill Gates video https://www.ted.com/talks/bill_gates_the_next_outbreak_we_re_not_ready/discussion?share=1fc9638c9c.
- University of Göttingen. "COVID-19: On average only 6% of actual SARS-CoV-2 infections detected worldwide: Actual number of infections may already have reached several tens of millions." ScienceDaily. ScienceDaily, 6 April 2020. <www.sciencedaily.com/releases/2020/04/200406125507.htm> <https://www.uni-goettingen.de/en/3240.html?id=5856>
- Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT, Fu H, Dighe A, Griffin JT, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon D, Nedjati-Gilani G, Riley S, van Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly CA, Ghani AC, Ferguson NM. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020 Mar 30. pii: S1473-3099(20)30243-7. doi: 10.1016/S1473-3099(20)30243-7. [Epub ahead of print] PubMed PMID: 32240634.
- Millett P, Snyder-Beattie A. Existential Risk and Cost-Effective Biosecurity. Health Security, 15, 4, 2017